On 27 May 2021, All.Can International launched its latest policy report Harnessing data for better cancer care.
The report assesses the essential role of data in cancer care to improve outcomes for all people with cancer. It offers policymakers, care providers, patients and decision-makers a forward-looking view of how to ensure high-quality health data are systematically collected and used to improve outcomes for patients across the entire cancer care pathway.
Download the full report here.
Download a report summary here.
What is the role of data in driving efficiency in cancer care? An All.Can policy paper
All.Can has developed a policy paper that takes a forward looking view on how we can evolve our health care systems and policy frameworks to enable data to drive greater efficiency in cancer care.
Why this paper?
Data are a key driver of efficiency: they underpin a learning healthcare system and can guide better use of resources to achieve better outcomes for patients.
The COVID-19 pandemic has magnified the value of data collection and sharing in oncology. But it has also exposed the fact that we are still far from the having an ideal, ready-to-use data system that can inform best practice.
Addressing existing gaps in our data ecosystem is a matter of urgency if we want to ensure the long-term sustainability of healthcare systems.
The paper was launched on 27 May 2021 on the occasion of the All.Can Global Summit.
Our research question?
The key research question this policy paper is trying to address is: How can the collection and use of data improve or potentially improve the efficiency of cancer care?
But it also looks into:
- How are data currently used along the patient care pathway?
- How could data contribute to greater efficiency at each stage of the pathway?
- What does the evidence tell us in terms of the role of data in driving efficiency in cancer care?
- What are some of the key challenges with the current use of data and how can they be addressed?
- What key recommendations can be made to guide policy change that will create an enabling environment for the optimisation of data in cancer care?
Aims and Objectives
We define data as:
… Any data describing a person’s health, their healthcare or anything affecting any health issues or diseases they may have. This includes information created by health and care professionals, as well as information generated by patients; from illnesses monitored through mobile applications and smart devices, to screening tests and nutritional data.
Source: https://datasaveslives.eu/health-data-overview
Which data we are focused on?
For purposes of feasibility, we are focused on types of data deemed of most relevance in cancer, namely: patient-generated data, including PROMs and PREMs; registry data; data from electronic health records and medical records; and genomics data.

How are we defining efficiency?
We have analysed efficiency through the lens of the patient, looking at the use and potential of data to drive efficiency at each stage of the care pathway, from prevention to survivorship to end-of-life care.
C ase studies
ase studies
We have identified 15 case studies showing projects, programmes or initiatives where data have improved outcomes and efficiency for patients that can be replicated elsewhere.
 Geographic reach
Geographic reach
The review focuses on Europe, with case studies drawn internationally.
The policy paper is based on a structured literature review, stakeholder interviews and case studies.
It has been developed with guidance from the All.Can Data Working Group
and an external advisory committee (please see Project governance).
For more information, please refer to the research methodology.
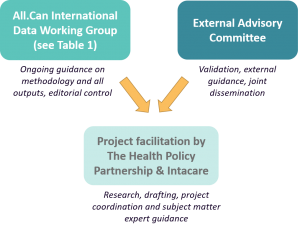
The report has been developed by The Health Policy Partnership and The Health Value Alliance on behalf of All.Can International.
The day-to-day guidance of this report was led by the Data Report Working Group, with expert advice and input from the External Advisory Committee.
All.Can International Data Report Working Group members
Sangeeta Agrawal, Helpsy
Antonella Cardone, European Cancer Patient Coalition
Ivana Cattaneo, Novartis
Dave Duplay, Vital Options International
Caroline Falciola, F. Hoffmann-La Roche
Alex Filicevas, World Bladder Cancer Patient Coalition, All.Can President
Stefan Gijssels, Digestive Cancers Europe
Dr. Hadi Mohamed Abu Rasheed, Qatar Cancer Society
Petra Hoogendoorn, Goings-On
Agnieszka Krukowska, Johnson & Johnson
Laura McDonald, Bristol Myers Squibb
Jan van Meerbeeck, Antwerp University Hospital
Matthijs Van Meerveld, Merck Sharp & Dohme
Borna Muller, F. Hoffmann-La Roche
Vivek Muthu, Marivek Consulting
Kathy Oliver, International Brain Tumour Alliance
Titta Rosvall-Puplett, Bristol Myers Squibb
Christobel Saunders, University of Western Australia
Julian Shepelev, Baxter Healthcare
Puneet Singhal, Merck Sharpe & Dohme
Henriette Thole, Novartis
Veronica Zilli, Johnson & Johnson
Matthew Hickey, The Health Value Alliance
Shannon Boldon, The Health Policy Partnership
Suzanne Wait, The Health Policy Partnership
External Advisory Committee
Fatima Cardoso, Advanced Breast Cancer Global Alliance
Sybo Dijkstra, DigitalEurope
Nigel Hughes, European Health Data and Evidence Network
Adrian Jonas, National Institute for Health and Care Excellence
Sabrina Montante, Istituto Superiore di Sanità
Ray Pinto, DigitalEurope
David Roder, University of South Australia
Abdullahi Sheriff, Merck Sharpe & Dohme
Francesco Pignatti, European Medicines Agency (observation only)
Independent Contributors
Miriam Gargesi, Illumina
Dipak Kalra, European Institute for Innovation through Health Data
Gregory Katz, University of Paris School of Medicine, PromTime
Steve Laws, Varian Medical Systems
Volker Liebenberg, Illumina
Laura O’Hanlon, Myriad Genetics
